A) ![]() K
K
B) ![]() K
K
C) ![]() P
P
D) ![]() P
P
E) ![]() K
K
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the metalloid in the following list.
A) sulfur
B) fluorine
C) silver
D) copper
E) germanium
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the electron arrangement for the atom shown. -Argon
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ionization energy is ________.
A) the energy an ion acquires from an electron
B) the energy needed to remove the least tightly bound electron
C) highest for metals in Group 1A (1)
D) higher for potassium than for lithium
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gives the correct numbers of protons,neutrons,and electrons in a neutral atom of 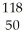 Sn?
Sn?
A) 118 protons,50 neutrons,118 electrons
B) 118 protons,118 neutrons,50 electrons
C) 50 protons,68 neutrons,50 electrons
D) 68 protons,68 neutrons,50 electrons
E) 50 protons,50 neutrons,50 electrons
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider an isotope of sodium with a mass number of 25.The number of neutrons in this isotope of sodium is ________.
A) 11
B) 14
C) 16
D) 25
E) 32
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct symbol for the element iron.
A) Ir
B) Fs
C) Fe
D) In
E) FE
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the electron arrangement for the atom shown. -The symbol for copper is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct symbol for the element silver.
A) S
B) Si
C) Ag
D) Au
E) AG
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element in this list with chemical properties similar to magnesium is ________.
A) sodium
B) boron
C) carbon
D) strontium
E) chlorine
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elements lithium,sodium,and potassium ________.
A) are isotopes of each other
B) are in the same period of elements
C) have the same number of neutrons
D) are in the same group
E) have the same mass number
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Short Answer
Write the electron arrangement for the atom shown. -The symbol for sodium is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the correct electron arrangement for the lithium atom?
A) 3
B) 3,1
C) 1,2
D) 2,1
E) 2,5
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is a noble gas?
A) oxygen
B) chlorine
C) bromine
D) argon
E) nitrogen
G) B) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The atomic number is equal to the number of protons.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
The symbol for potassium is P.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of chlorine has two naturally occurring isotopes.The isotope Cl-35 makes up 75.8% of the sample,and the isotope Cl-37 makes up 24.3% of the sample.Which of the following statements is true?
A) The atomic mass of chlorine will be less than 35.
B) The atomic mass of chlorine will be more than 37.
C) You cannot tell what the atomic mass will be.
D) The atomic mass will be between 35 and 37.
E) The atomic mass will be 24.3.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Isotopes are atoms of the same element that have ________.
A) different atomic numbers
B) the same atomic numbers but different numbers of protons
C) the same atomic numbers but different numbers of electrons
D) the same atomic number but different numbers of neutrons
E) the same atomic mass but different numbers of protons
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ionization energy of atoms ________.
A) decreases going across a period
B) decreases going down within a group
C) increases going down within a group
D) does not change going down within a group
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
Sulfur is a nonmetal.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 106
Related Exams