A) C4H14
B) C3H21
C) C2H7
D) C2H14
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
The empirical formula mass is 18.0 and the molecular formula mass is 90,therefore n = 5.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
Avogadro's Number is 6.022 × 1023.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of Pb are in 4.71 × 1021 Pb atoms?
A) 0.00782
B) 2.84 × 1045
C) 207.2
D) 6.022 × 1023
E) none of the above
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms of lithium are in 18.7 g?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles are there in 82.5 grams of iron?
A) 4.97 × 1025
B) 55.85
C) 0.677
D) 1.48
E) none of the above
G) All of the above
Correct Answer

verified
Correct Answer
verified
True/False
The mass of 2.0 moles of H2O is greater than the mass of 1.0 mole of CO2.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of ammonium carbonate.
A) 78.05 g/mol
B) 88.05 g/mol
C) 96.09 g/mol
D) 112.09 g/mol
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
True/False
An empirical formula gives the smallest whole number ratio of each type of atom in a molecule.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of calcium nitrate.
A) 136.03 g/mol
B) 102.09 g/mol
C) 132.10 g/mol
D) 164.10 g/mol
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
True/False
One mole of chlorine gas has a mass of 35.45 grams.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has the highest mass percent of "O"?
A) MnO
B) MnO2
C) Mn2O3
D) Mn3O2
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many atoms are in 5.80 moles of He?
A) 6.02 × 1023
B) 1.03 × 1023
C) 4.00
D) 3.49 × 1024
E) none of the above
G) B) and D)
Correct Answer

verified
Correct Answer
verified
True/False
Water is 11.2% hydrogen by mass.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 15.5 gram sample of diphosphorous pentoxide contains how many grams of phosphorous?
A) 3.38
B) 1.69
C) 6.76
D) 13.5
E) none of the above
G) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If 3.011 ×
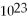 Molecules have a mass of 20.04 grams,what is the molar mass of this substance?
Molecules have a mass of 20.04 grams,what is the molar mass of this substance?
A) 40.08 g/mol
B) 10.02 g/mol
C) 20.04 g/mol
D) 6.658 × ![]()
G/mol
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
True/False
The empirical formula for C6H6 is C3H3.
B) False
Correct Answer

verified
Correct Answer
verified
True/False
An empirical formula gives the specific number of each type of atom in a molecule.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of oxygen gas has a mass of ________ g.
A) 16.0
B) 32.0
C) 6.022 × 1023
D) 8
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular formula of a compound given the molar mass of the compound is 30.04 gram and the empirical formula is NH?
A) NH
B) N2H2
C) N2H6
D) N4H4
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 118
Related Exams