A) C22H34O10
B) C6H6
C) C6H12O3
D) C5H12O2
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
A molecule that has an empirical formula of HO and a molar mass of 34.02 gram must have a molecular formula of H2O2.
B) False
Correct Answer

verified
True
Correct Answer
verified
True/False
One mole of argon has more atoms in it than one mole of neon.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of (NH4) 2HPO4 contains how many moles of hydrogen atoms?
A) 4
B) 2
C) 8
D) 9
E) none of the above
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the mass percent of carbon in oxalic acid,H2C2O4?
A) 2.24
B) 13.3
C) 26.7
D) 34.5
E) none of the above
G) B) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The molar mass of a compound in grams per mole is numerically equal to the formula mass of the compound in atomic mass units.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of boron has a mass of ________ g.
A) 9.012
B) 6.022 × 1023
C) 5
D) 10.811
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the molar mass of calcium nitrate.
A) 136.03 g/mol
B) 102.09 g/mol
C) 132.10 g/mol
D) 164.10 g/mol
E) none of the above
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many molecules of sulfur trioxide are in 78.0 grams?
A) 5.87 × 1023
B) 7.33 × 1023
C) 3.76 × 1027
D) 0.974
E) none of the above
G) D) and E)
Correct Answer

verified
A
Correct Answer
verified
True/False
One mole of lead(II)nitrate contains six moles of oxygen atoms.
B) False
Correct Answer

verified
True
Correct Answer
verified
Multiple Choice
How many moles of Pb are in 4.71 × 1021 Pb atoms?
A) 0.00782
B) 2.84 × 1045
C) 207.2
D) 6.022 × 1023
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a sample of carbon dioxide contains 3.8 moles of oxygen atoms,how many moles of carbon dioxide are in the sample?
A) 1.9
B) 3.8
C) 7.6
D) 11.4
E) none of the above
G) A) and B)
Correct Answer

verified
Correct Answer
verified
True/False
One mole of water contains 6.022 × 1023 hydrogen atoms.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the empirical formula of a compound containing 83% potassium and 17.0% oxygen.
A) KO
B) KO2
C) K2O3
D) K2O
E) none of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The empirical formula for C6H6 is C3H3.
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of iron are contained in 1.75 kg of iron?
A) 3.13 × 10-2
B) 3.13 × 10-4
C) 31.3
D) 3.13 × 104
E) none of the above
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One mole of oxygen gas has a mass of ________ g.
A) 16.0
B) 32.0
C) 6.022 × 1023
D) 8
E) none of the above
G) A) and C)
Correct Answer

verified
Correct Answer
verified
True/False
The correct formula for calculating mass percent of X in compound XY is: 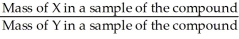 = Mass % X
= Mass % X
B) False
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In comparing 1 mole of carbon atoms to one mole of magnesium atoms,which statement is TRUE?
A) The mass of 1 mole of carbon is greater than the mass of 1 mole of magnesium.
B) The mass of 1 mole of magnesium is greater than the mass of 1 mole of carbon.
C) The mass of 1 mole of carbon is the same as the mass of 1 mole of magnesium.
D) There are more atoms in 1 mole of magnesium than in 1 mole of carbon.
E) none of the above
G) D) and E)
Correct Answer

verified
Correct Answer
verified
True/False
The mole has a value of 6.023 × 1022.
B) False
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 118
Related Exams