A) a compound with the same number of carbon atoms as the hormone
B) a compound with the same molecular mass (measured in daltons) as the hormone
C) a compound with the same three-dimensional shape as part of the hormone
D) a compound with the same number of orbital electrons as the hormone
E) a compound with the same number of hydrogen and nitrogen atoms as the hormone
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not considered to be a weak molecular interaction?
A) a covalent bond
B) a van der Waals interaction
C) an ionic bond in the presence of water
D) a hydrogen bond
E) A and B only
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Short Answer
Please refer to Figure 2.1 to answer the following questions.
 -Which drawing is of an atom with the atomic number of 6?
-Which drawing is of an atom with the atomic number of 6?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules contains the strongest polar covalent bond?
A) H₂
B) O₂
C) CO₂
D) H₂O
E) CH₄
G) B) and D)
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
H is a radioactive isotope of hydrogen. One difference between hydrogen-1 ( H) and hydrogen-3 ( H) is that hydrogen-3 has
A) one more neutron and one more proton than hydrogen-1.
B) one more proton and one more electron than hydrogen-1.
C) one more electron and one more neutron than hydrogen-1.
D) two more neutrons than hydrogen-1.
E) two more protons than hydrogen-1.
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What coefficients must be placed in the following blanks so that all atoms are accounted for in the products? C₆H₁₂O₆ → ___ C₂H₆O + ___ CO₂
A) 1; 2
B) 2; 2
C) 1; 3
D) 1; 1
E) 3; 1
G) C) and D)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
The reactivity of an atom arises from
A) the average distance of the outermost electron shell from the nucleus.
B) the existence of unpaired electrons in the valence shell.
C) the sum of the potential energies of all the electron shells.
D) the potential energy of the valence shell.
E) the energy difference between the s and p orbitals.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ionic bond of sodium chloride is formed when
A) chlorine gains an electron from sodium.
B) sodium and chlorine share an electron pair.
C) sodium and chlorine both lose electrons from their outer valence shells.
D) sodium gains an electron from chlorine.
E) chlorine gains a proton from sodium.
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
One difference between carbon-12 ( C) and carbon-14 ( C) is that carbon-14 has
A) two more protons than carbon-12.
B) two more electrons than carbon-12.
C) two more neutrons than carbon-12.
D) A and C only
E) B and C only
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom with an atomic number of 9 and a mass number of 19 would have an atomic mass of approximately
A) 9 daltons.
B) 9 grams.
C) 10 daltons.
D) 20 grams.
E) 19 daltons.
G) A) and D)
Correct Answer

verified
E
Correct Answer
verified
Short Answer
Please refer to Figure 2.1 to answer the following questions.
 -Which drawing depicts an atom with a valence of 3?
-Which drawing depicts an atom with a valence of 3?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an atom of sulfur (atomic number 16) were allowed to react with atoms of hydrogen (atomic number 1) , which of the molecules below would be formed?
A) S- H
B) H- S -H
C) ![]()
D) ![]()
E) H=S=H
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Atoms can be represented by simply listing the number of protons, neutrons, and electrons for example, 2p⁺; 2n⁰; 2e⁻ for helium. Which one of the following lists represents the 18O isotope of oxygen?
A) 6p⁺; 8n⁰; 6e⁻
B) 8p⁺; 10n⁰; 8e⁻
C) 9p⁺; 9n⁰; 9e⁻
D) 7p⁺; 2n⁰; 9e⁻
E) 10p⁺; 8n⁰; 9e⁻
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Short Answer
Please refer to Figure 2.1 to answer the following questions.
 -Which drawing depicts an atom with a valence of 2?
-Which drawing depicts an atom with a valence of 2?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the relationship between the atoms described below?
A) They are isomers.
B) They are polymers.
C) They are isotopes.
D) They contain 1 and 3 protons, respectively.
E) They each contain 1 neutron.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the following figure to answer the following questions.
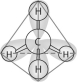 -Which of the following pairs of atoms would be most likely to form an ionic bond?
-Which of the following pairs of atoms would be most likely to form an ionic bond?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following explains most specifically the attraction of water molecules to one another?
A) nonpolar covalent bond
B) polar covalent bond
C) ionic bond
D) hydrogen bond
E) hydrophobic interaction
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each element is unique and different from other elements because of the number of protons in the nuclei of its atoms. Which of the following indicates the number of protons in an atom's nucleus?
A) atomic mass
B) atomic weight
C) atomic number
D) mass weight
E) mass number
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
About 25 of the 92 natural elements are known to be essential to life. Which four of these 25 elements make up approximately 96% of living matter?
A) carbon, sodium, chlorine, nitrogen
B) carbon, sulfur, phosphorus, hydrogen
C) oxygen, hydrogen, calcium, sodium
D) carbon, hydrogen, nitrogen, oxygen
E) carbon, oxygen, sulfur, calcium
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the information extracted from the periodic table in Figure 2.2 to answer the following questions.
 -What is the maximum number of electrons in a 2p orbital of an atom?
-What is the maximum number of electrons in a 2p orbital of an atom?
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 83
Related Exams